Topics Covered
- Why MRO Data Is the Hidden Driver of Plant Reliability
- The Poor Data Cycle: A Repeating Pattern of Waste
- Breaking the Poor Data Cycle:
- From Chaos to Clarity: Standardization and Global Visibility
- RS SYNC™ Turning Data into Strategic Action
- Smarter Procurement: From Reactive to Strategic
- Sustainable Savings and ESG Alignment
- Access and Availability: The Ultimate Goal
- Frequently asked questions
- Ready to Fix Your MRO Data?
Tags
Published on
Read time: 9 minutes

In pharmaceutical manufacturing, every minute of downtime can cost thousands in lost productivity, regulatory risk, and missed market opportunities. Yet one of the most persistent and costly issues in the supply chain isn’t a broken machine or a delayed shipment. It is bad data
Why MRO Data Is the Hidden Driver of Plant Reliability
Maintenance, Repair, and Operations (MRO) materials are the lifeblood of plant reliability. But when the data behind those materials is inaccurate, incomplete, or duplicated, it creates a ripple effect of inefficiency across procurement, inventory, and engineering. The good news? This is a solvable problem and solving it unlocks significant value.
The Real Cost of Bad MRO Data
- Poor data leads to emergency purchases, expedited shipping costs, excess inventory, and wasted time
- Up to 70% of MRO data in ERP systems is inaccurate or unusable
- Clean data improves visibility, reduces downtime, and cuts unnecessary spend
MRO data lives in ERP and MRP systems like SAP, Oracle, and JD Edwards. But too often, it’s riddled with vague descriptions, missing part numbers, and inconsistent formatting. When an engineer searches for a part and can’t find it, they resort to ad hoc purchases, often at a premium. Worse, many of those emergency purchases end up unused, sitting in drawers or storerooms.
On average, 46% of client inventory exceeds actual demand, and 50% haven’t moved in over a year. That is not just inefficient, it is cash and it is sitting idle.
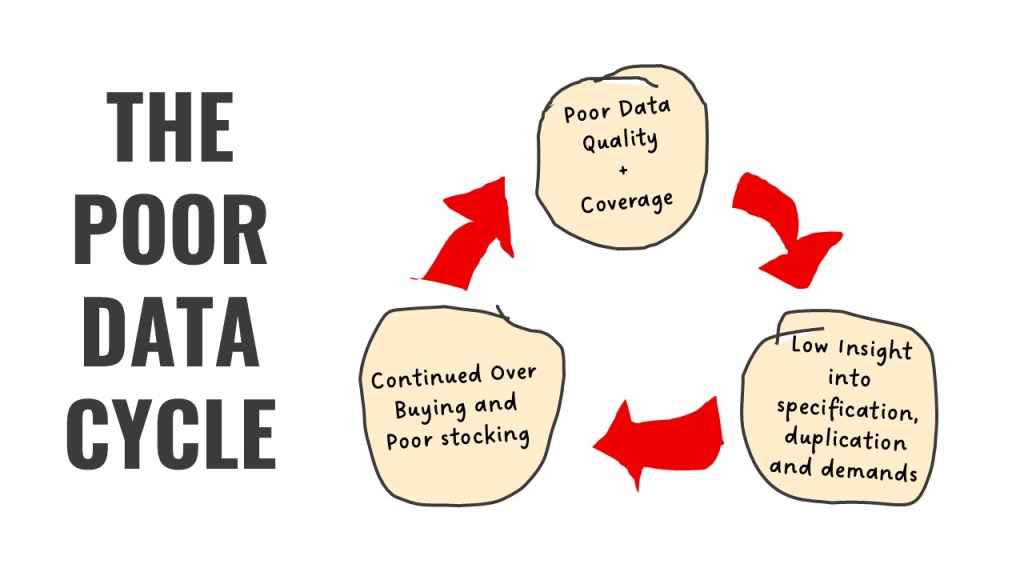
The Poor Data Cycle: A Repeating Pattern of Waste
- Traditional procurement methods reinforce bad data habits
- Rebates and sign-on bonuses mask deeper inefficiencies
- Without clean data, organizations overbuy, duplicate, and mismanage inventory
Many procurement teams follow a familiar pattern: issue a tender, send out a market basket, negotiate rebates, and secure sign-on bonuses. But this process often ignores the root problem, data quality. “What you want to do is avoid what we call the poor data cycle,” says Simon Hilton, Global Senior Vice President for Business Development and Marketing at RS Integrated Supply. “That cycle starts with bad data, leads to low insight, and results in overstocking and misaligned purchases. The rebate may look good on paper, but the underlying inefficiencies remain.”
Breaking the Poor Data Cycle:
Cleaning the Data: AI and Human Collaboration
- AI can assist in cleaning and standardizing up to 60% of poor data
- Human validation is still required for nuance and accuracy
- Combining AI and human review achieves near-complete data integrity
Modern tools can dramatically improve MRO data quality. AI algorithms scan descriptions, identify manufacturer part numbers, and classify items into categories like hydraulics, pneumatics, and power transmission. But AI alone isn’t enough. Humans still need to resolve ambiguities like color codes, banding, and specific terminology.
RS Integrated Supply uses a hybrid approach with AI and human validation. This combination speeds up the process by 75% and delivers up to 100% clean data.
“We verify the data that AI reviewed. What is not correct, we use additional mobile technology and go and inspect the part and identify it properly.”
-Simon Hilton, SVP, RS Integrated Supply
“We completed one client storeroom just recently with about 28,000 parts and followed this process,” said Hilton. “We verify the data that AI reviewed. What is not correct, we use additional mobile technology and go and inspect the part and identify it properly.”
From Chaos to Clarity: Standardization and Global Visibility

- Clean data enables standardization across sites and suppliers
- Duplicate SKUs are eliminated, reducing complexity and cost
- A single global ID allows for real-time visibility and smarter procurement
Once data is clean, organizations can standardize specifications across multiple sites. One recent example from RS Integrated Supply involved PTFE tape, which appeared under 55 different part numbers across 38 locations. The cleansed data reduced that information to a single specification, one vendor, and one part number, globally.
This standardization unlocks procurement leverage. Teams can negotiate better terms, reduce supplier fragmentation, and ensure consistency across operations.
RS SYNC™ Turning Data into Strategic Action
- RS SYNC™ centralizes and standardizes MRO data across global operations.
- The RS SYNC™ Marketplace connects users to a curated network of suppliers and inventory.
- Engineers and buyers gain real-time access to availability, pricing, and alternatives.
Clean data is only powerful if it is accessible and actionable. Having the proper technology to manage the process and leverage the outputs is also essential. “Everybody has data but not everyone can turn that data into information,” said Hilton. “In most systems there will be thousands of lines of all different types of master data around spare parts but it’s not yet information. You must turn that data into information before you can benefit from the insight you gain.”
“Everybody has data but not everyone can turn that data into information.”
-Simon Hilton, SVP, RS Integrated Supply
RS Integrated Supply clients gain access to the company’s proprietary RS SYNC™ technology, a platform that serves as a centralized hub for MRO data, integrating with existing ERP systems while enhancing visibility and control.
Through RS SYNC™ Marketplace clients can search for parts using a global ID, check availability across their network, and even receive instant quotes from preferred suppliers, streamlining procurement and reducing spend without compromising reliability.
All because of clean data.
Smarter Procurement: From Reactive to Strategic
- Clean data enables category planning based on real usage
- Risk is reduced by ensuring the availability of critical parts
- Engineers can see availability, pricing, and alternatives in real-time
Knowing what’s critical and what’s not is essential. Clean data allows procurement teams to build category strategies around consumption patterns. It also reduces risk by ensuring that essential parts are always available, even if they are not the cheapest.
With standardized data, manufacturers can assign global IDs to parts. This enables real-time visibility across locations. Engineers can search for a part, check if stock availability, and receive instant quotes. For non-critical items, systems can even suggest lower-cost alternatives, streamline procurement, and reduce spend.
“The point of all of this is to speed up the process of getting the product into the hands of the engineer or maintenance person when the plant needs to keep running.,” Hiltons says.
Engineers no longer need to hoard parts – “just in case” – they can trust the system to deliver what’s needed. This reduces excess stock, frees up shelf space, and lowers overall inventory value. It also means different sites within a company can share parts when needed, particularly those with longer lead times, which further improves downtime metrics.
Sustainable Savings and ESG Alignment
- Clean data enables the selection of energy-efficient, longer-lasting parts
- EHS-compliant and lower-carbon alternatives can be prioritized
- Future-proofing the supply chain supports ESG goals
Sustainability is increasingly important in pharma and life sciences. Clean data allows companies to identify and switch to “better world” products, those that use less energy, last longer, or have lower environmental impact. This aligns procurement with broader ESG initiatives and regulatory requirements.
Access and Availability: The Ultimate Goal
- Engineers need instant visibility into what’s available and where
- Clean data eliminates panic buying and downtime
- The goal is to keep manufacturing running efficiently and reliably
In interviews with maintenance and engineering teams, two words came up repeatedly: access and availability. Clean data delivers both. It ensures that the right part is in the right place at the right time, reducing downtime, improving planning, and eliminating the need for emergency purchases.
The future of MRO isn’t about chasing rebates or negotiating market baskets. It is about building a better process, one that starts with clean data, enables visibility, and drives strategic value. With the right tools and mindset, MRO can become a competitive advantage.
Frequently asked questions
MRO data refers to the information tied to the spare parts and materials that keep your plant running, everything from valves and sensors to safety gear and lubricants. In pharmaceutical manufacturing, where uptime is critical and compliance is non-negotiable, clean MRO data is essential. It ensures that engineers can quickly find the right part, procurement teams can make informed decisions, and operations stay efficient and audit ready. Without accurate data, even the best ERP system can become a bottleneck.
Bad MRO data creates a ripple effect of inefficiency. When part numbers are missing, descriptions are vague, or duplicates exist, engineers can’t find what they need and that leads to emergency purchases, excess inventory, and production delays. Clean data, on the other hand, improves visibility, reduces risk, and helps keep the plant running smoothly.
MRO data tends to fly under the radar because it is not part of the direct production process, it is indirect, but still essential. During ERP rollouts or digital transformation projects, the focus is often on core production activity. As a result, little attention is given to MRO data and it is migrated into the system “as-is.” That means all the inconsistencies, duplicates, and gaps come along for the ride. Ironically, clean MRO data is one of the fastest ways to unlock value from your ERP investment. It is the foundation for smarter procurement, better inventory control, and improved plant reliability.
RS SYNC™ is a centralized procurement platform used in managing MRO transactions across your global operations. It integrates with your existing ERP system and gives engineers and buyers real-time access to part availability, pricing, and alternatives. It includes RS SYNC™ Marketplace through which users can search using a global ID, check inventory across sites, and even get instant quotes from preferred suppliers. It is a strategic enabler that turns clean data into actionable insight, helping you reduce spend, improve uptime, and future-proof your supply chain.
Standardized MRO data eliminates duplicate SKUs, aligns specifications across sites, and gives you a clear picture of what is in stock and what is not. That means no more hoarding “just in case” parts, no more emergency buys, and no more guessing. With clean, consistent data, you can optimize inventory levels, reduce carrying costs, and ensure critical parts are always available when needed.
Ready to Fix Your MRO Data?
RS Integrated Supply helps pharmaceutical and life sciences companies clean their data, optimize inventory, and future-proof their supply chains. If you are ready to move beyond rebates and into real value, request a consultation today.





